ASCRS 2025 | Dual Clinical Results of Eyedeal® Cross-Linked Polyisobutylene (xPIB) Intraocular Lens Unveiled
On April 27, the third day of the 2025 Annual Meeting of the American Society of Cataract and Refractive Surgery (ASCRS), Xi’an Eyedeal Medical Technology Co., Ltd. presented two major clinical research findings on its innovative medical device — the Eyedeal® Cross-Linked Polyisobutylene (xPIB) Intraocular Lens (IOL) — during the “Monofocal and Multifocal IOLs” session. These results highlighted the product’s outstanding performance in terms of safety, efficacy, and in-the-bag stability, garnering widespread praise from global ophthalmic experts and scholars.
Clinical Study 1
Randomized, Multi-Center Clinical Trial to Evaluate Safety and Efficacy of a Novel Cross-Linked Polyisobutylene (xPIB) IOL

At the forum, Professor Qun Peng, Chief Medical Officer of Eyedeal, presented the results of a randomized, multicenter clinical trial conducted in China to evaluate the Eyedeal® xPIB IOL. The study was led by the Eye Hospital of Wenzhou Medical University and jointly implemented by several top-tier ophthalmic centers including Xi’an People’s Hospital (Xi’an Fourth Hospital), the Eye Hospital Affiliated with Shandong University of Traditional Chinese Medicine, Xiangya Hospital of Central South University, and the Joint Shantou International Eye Center of Shantou University and the Chinese University of Hong Kong.

The clinical data showed that six months after cataract surgery, all participants implanted with the Eyedeal® xPIB IOL achieved best-corrected visual acuity (BCVA), confirming the device’s strong efficacy in visual restoration. Notably, at 12 months post-surgery, the mean BCVA approached 0 logMAR, indicating long-term stable visual quality. In terms of refractive accuracy, the Eyedeal® xPIB IOL demonstrated superior performance compared to the control product Acrysof® SN60WF (Alcon, USA), providing patients with clearer and more precise postoperative vision.
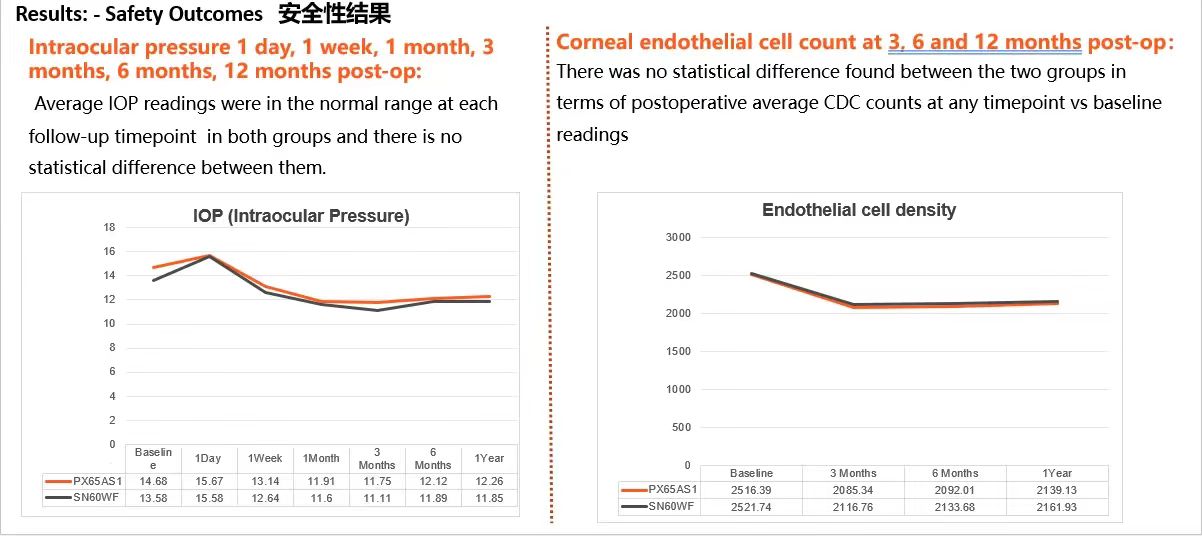
Regarding safety, the results were equally impressive. Throughout the 12-month follow-up period, no severe complications such as lens dislocation, decentration, tilt, infection, or device malfunction were reported.
Clinical Study 2
Clinical Evaluation of In-the-Bag Stability of a Novel IOL Made of Cross-Linked Polyisobutylene

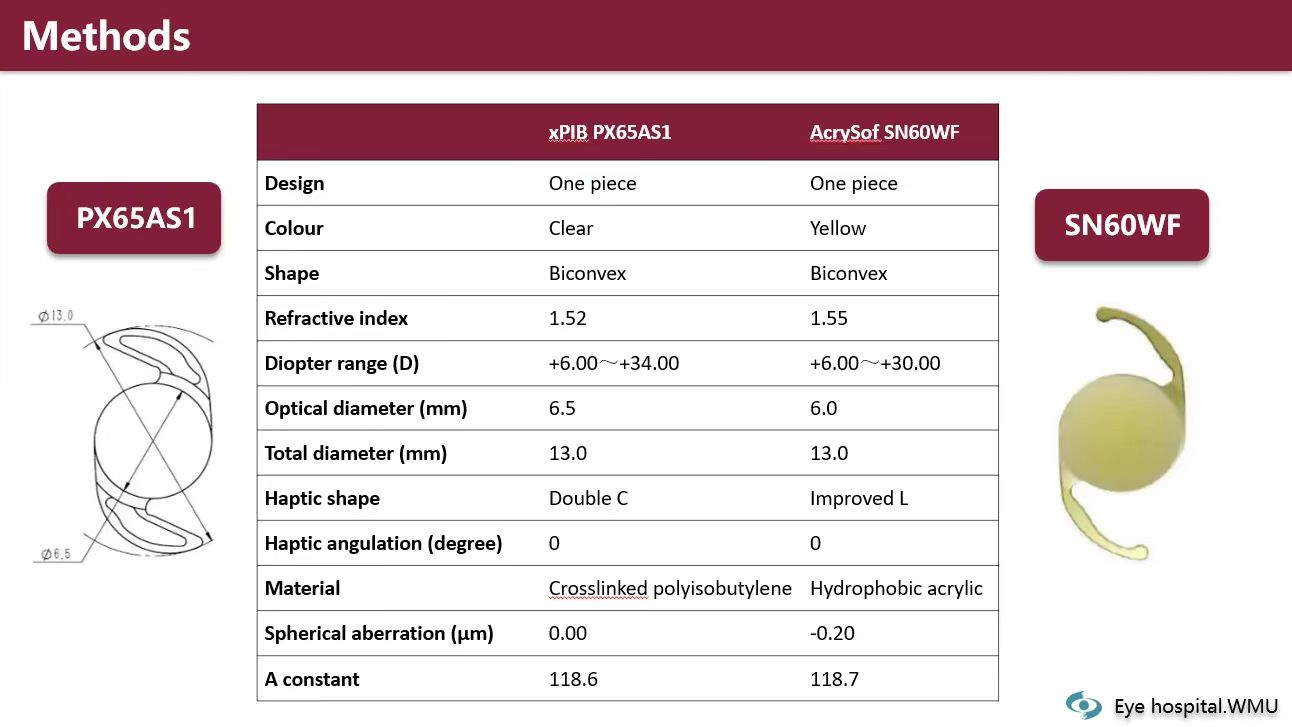
Professor Peng also shared findings from another clinical study conducted by Drs. Yinying Zhao and Yun’e Zhao at the Eye Hospital of Wenzhou Medical University. This prospective, randomized, parallel-controlled clinical trial aimed to assess the in-the-bag stability and visual recovery of the Eyedeal® xPIB IOL.

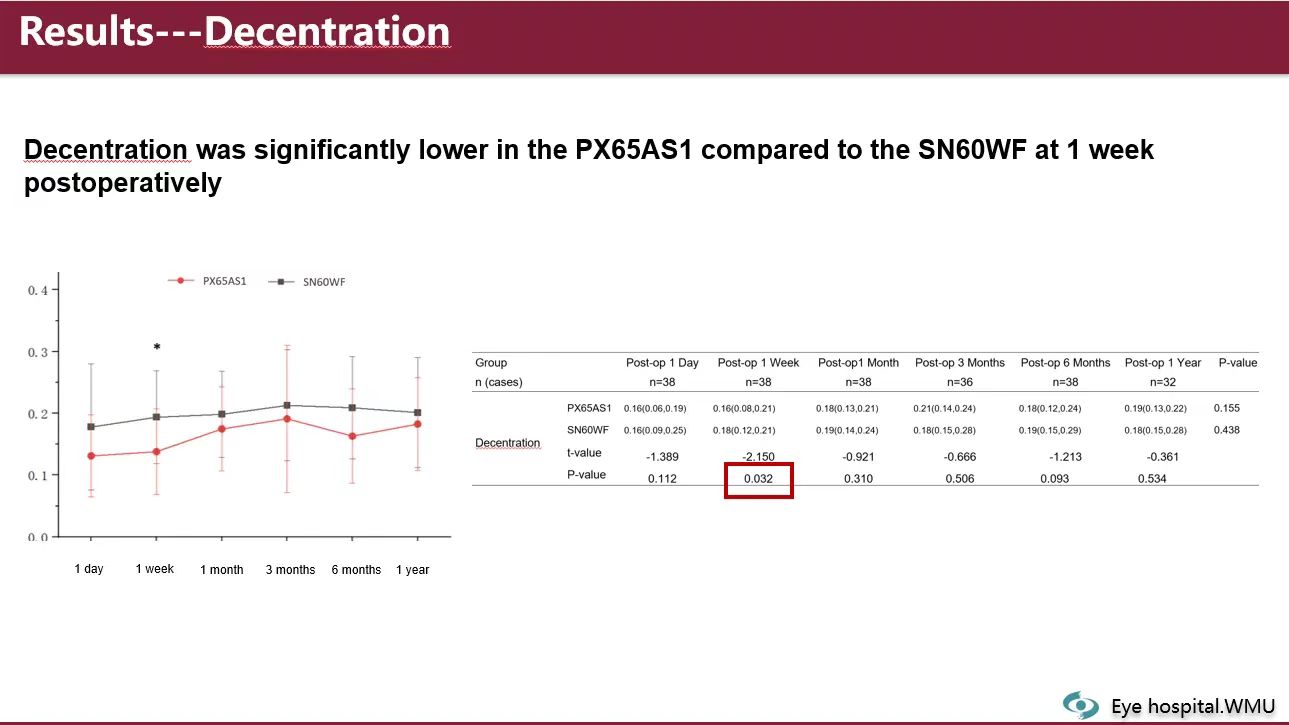
The data showed that just one week post-surgery, the decentration of the Eyedeal® xPIB IOL was significantly lower than that of the control group (Acrysof® SN60WF), suggesting superior early postoperative stability. Furthermore, across all follow-up intervals, there were no statistically significant differences between the Eyedeal® xPIB IOL group and the control group in key indicators such as uncorrected visual acuity (UCVA), BCVA, IOL tilt, or postoperative anterior chamber depth (PACD).
Overall, the Eyedeal® xPIB IOL not only achieved precise refractive correction but also maintained excellent in-the-bag positional stability, providing patients with high-quality and lasting visual outcomes.
After the presentations, international ophthalmic experts praised the innovation of the Eyedeal® xPIB IOL. They recognized its exceptional performance in safety, effectiveness, and stability, noting that these strengths lay a solid foundation for its future clinical adoption. Experts expressed great anticipation for the product’s early market availability, expecting it to deliver superior postoperative visual experiences for cataract patients worldwide.